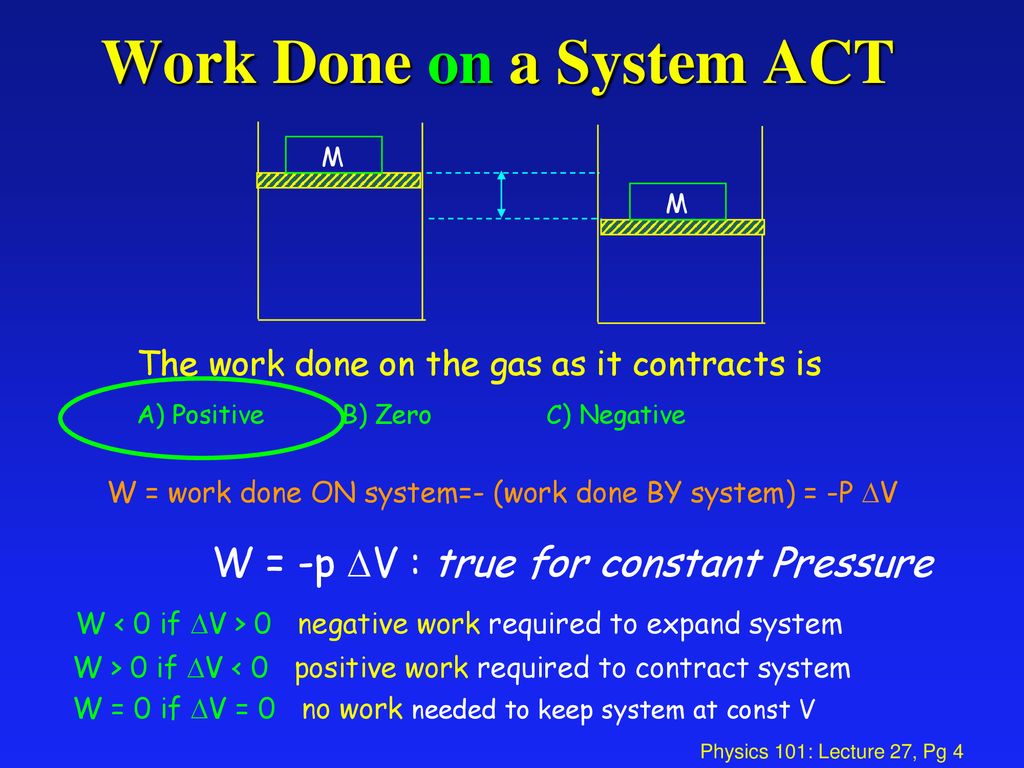
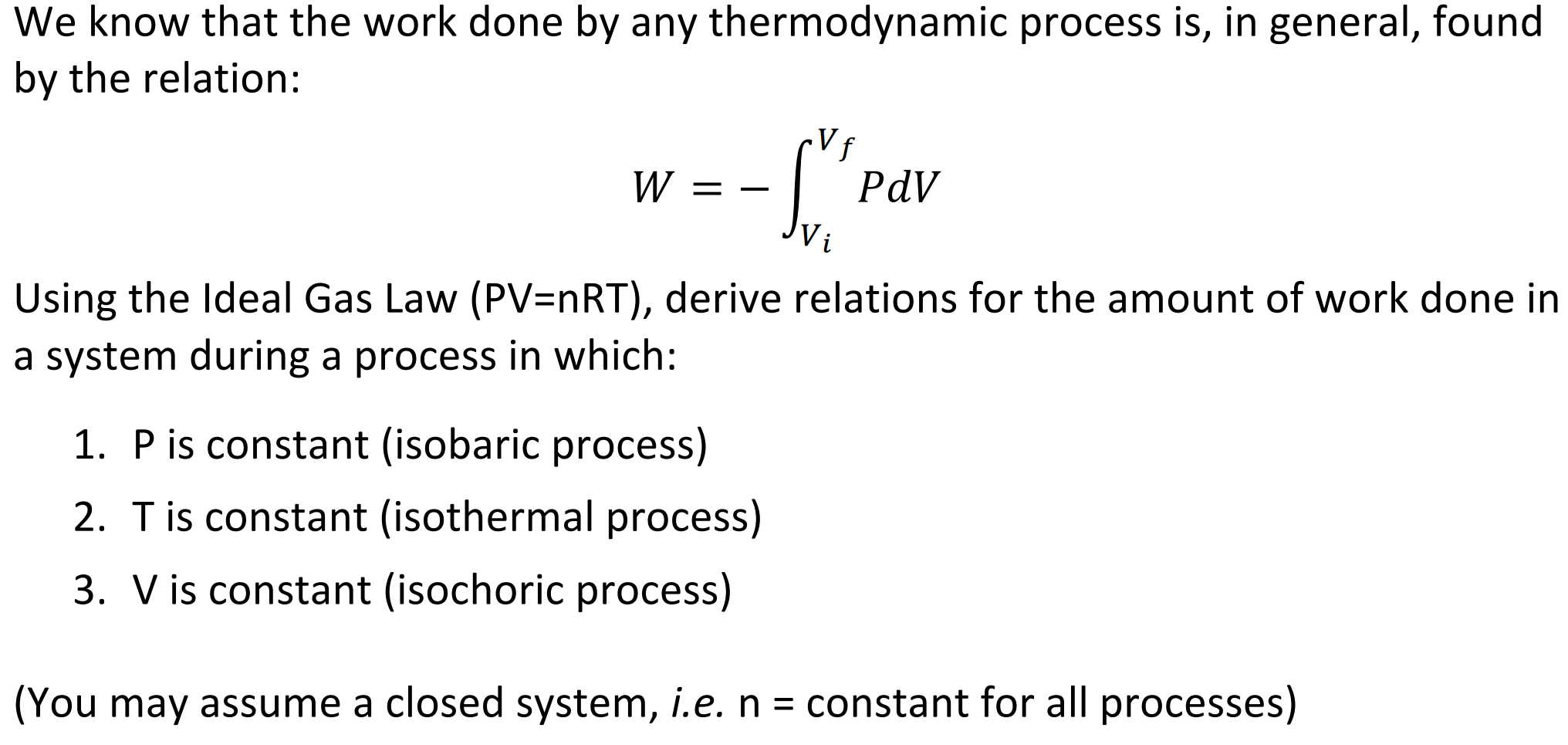
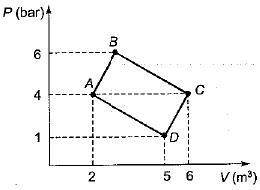
Work Done On The System
Work done on the system. In this channel of YouTube are edited videos for high school students as well as for students of physics chemistry biology medicine pharmacy agriculture. The work done by the system in an isobaric process is simply the pressure multiplied by the change in volume and the P-V graph looks like. What is the work in J done on the system.
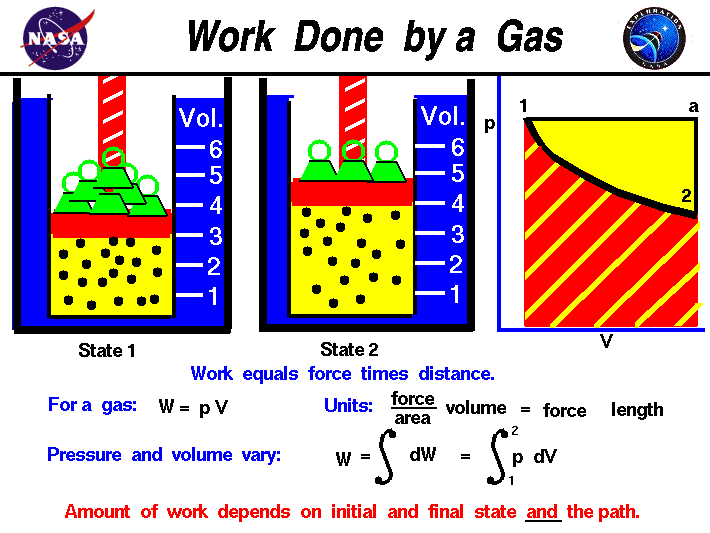
In thermodynamics work performed by a system is energy transferred by the system to its surroundings by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings. Help given in feedback. The total work done during a finite displacement of the piston is now easy to calculate.
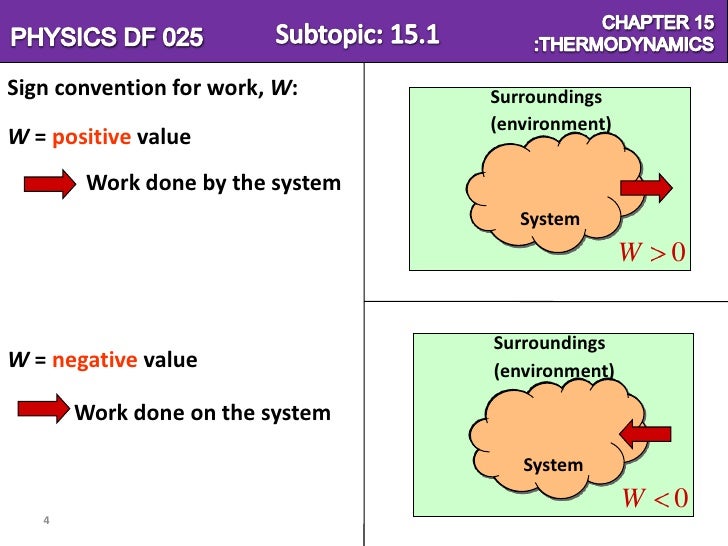
When N coulombs of electrons move through a potential difference V the electrical work done is. If you pick up the ball and place. Work is done BY the system during the power stroke ie.
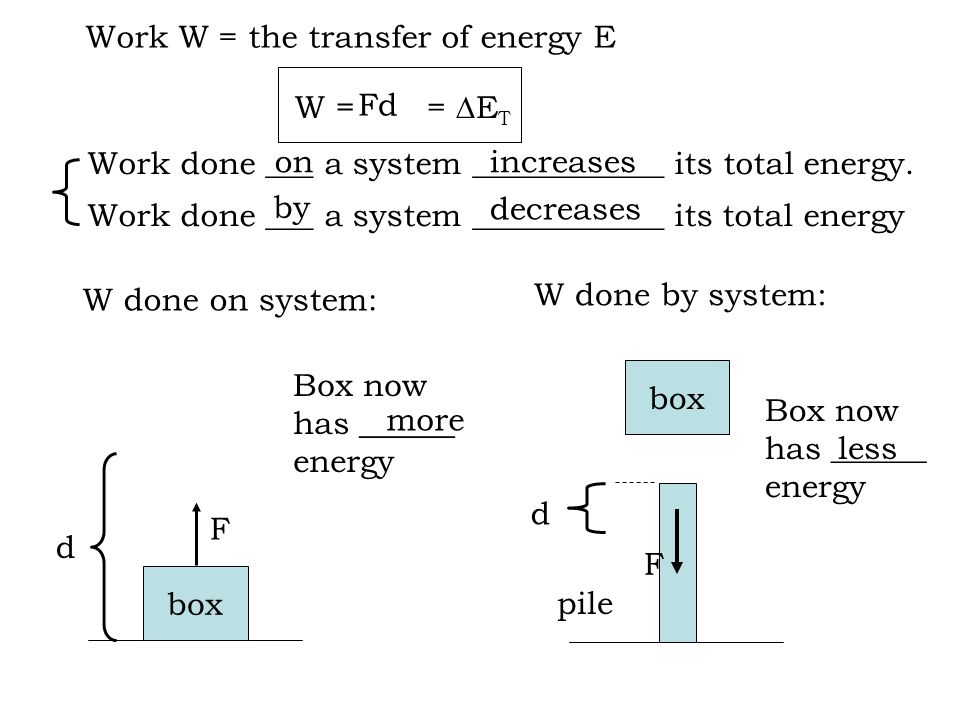
Work is a form of energy but it is energy in transit. Kinetic energy potential energy and internal energy are forms of energy that are properties of a system. W is the work done on or by the system 3 describe the.
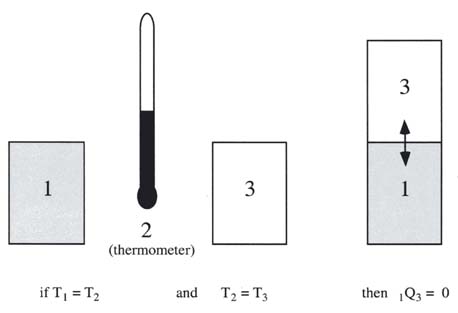
Q is negative if heat leaves the system. For example your system may be a tennis ball. What is AE in J.
In SI system unit of work is 1Nm and is given a name JouleJ. Consider a simple compressible substance Work done by system therefore. Strategy The machinists force over a distance that can be calculated from the speed and time given is the work done on the system.
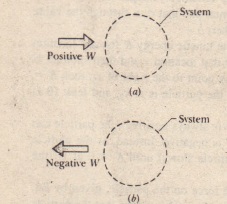
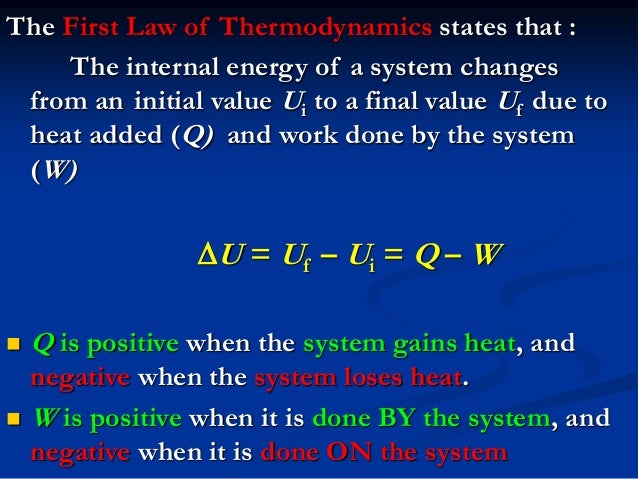
System Q W. W is the work done on or by the system.
If you pick up the ball and place.
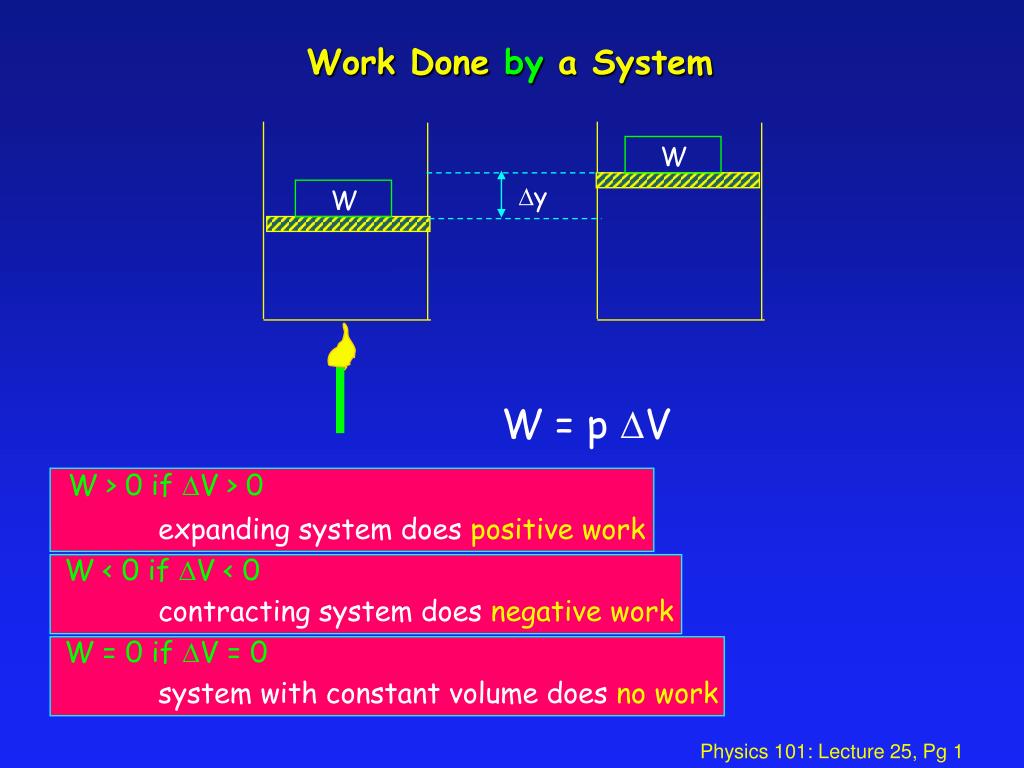
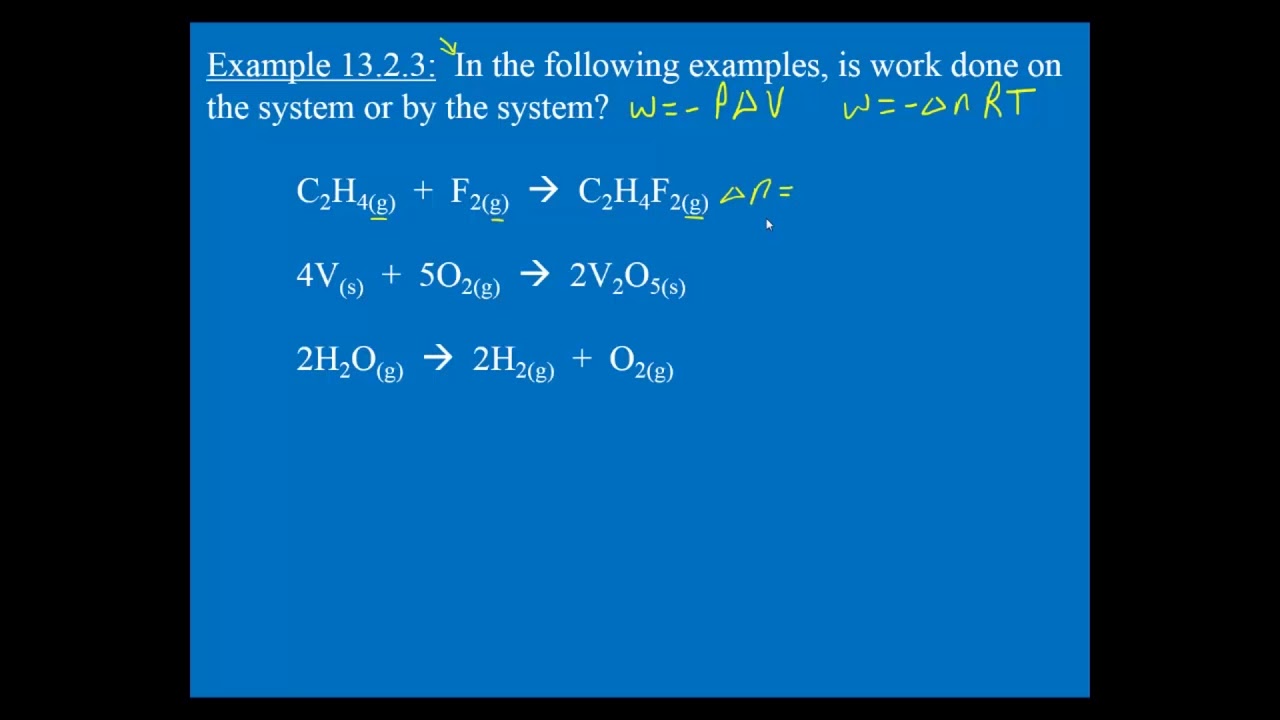
In SI system unit of work is 1Nm and is given a name JouleJ. Q is negative if heat leaves the system. Unit of work done in any system of units is equal to the unit of force multiplied by the unit of distance. For example your system may be a tennis ball. Work done on the system means something outside the system did something other than heat flow into the system to increase the internal energy of the system. The work in turn increases the internal energy of the system. The work done by the system in an isobaric process is simply the pressure multiplied by the change in volume and the P-V graph looks like. Electrical Work The work that is done on a system by electrons. 483 J of heat are added to the system.
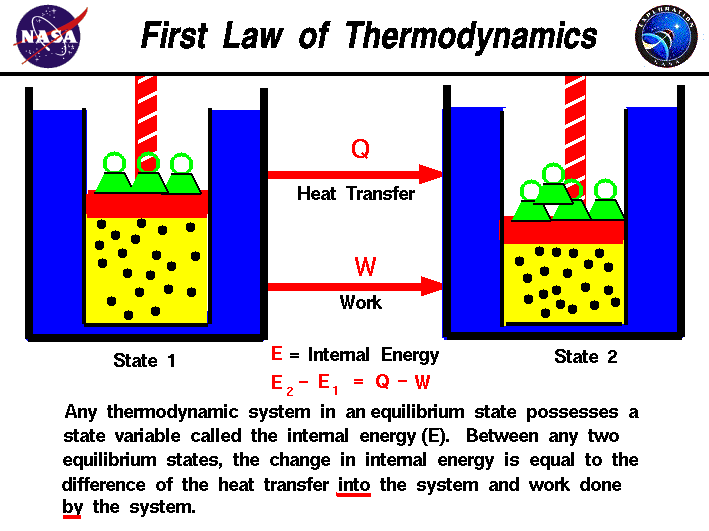
If the system has such rigid walls that pressurevolume work cannot be done but the walls are adiabatic Q 0 and energy is added as isochoric constant volume work in the form of friction or the stirring of a viscous fluid within the system W 0 and there is no phase change then the temperature of the system will rise. Help given in feedback. In the first case Delta U q - w while in the second case Delta U q w. The work done by the system in an isobaric process is simply the pressure multiplied by the change in volume and the P-V graph looks like. System work is a major focus in the discussion of heat engines. Or in terms of the specific volume v. In other words Equation refeq720 applies to system A as well as it does to system B even though the interaction between the parts that make up system A is dissipative.



















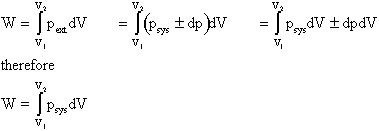





Post a Comment for "Work Done On The System"